Medical Device Development and Design
Innovative Ideas, Brought Into Being
At in2being, we specialize in helping both businesses and individuals navigate medical device development and design within the regulatory landscape in the most efficient way possible.
in2being’s skilled med-tech professionals are the catalyst that brings customer ideas to life while achieving clearance through the FDA medical device regulation process and other regulatory bodies.
Streamlined, Efficient, and Transparent FDA Clearance
Communicating your vision should be your primary focus. We can handle the rest.
Our staff is adept at guiding clients through the murky waters of the FDA clearance process for medical devices.
We leverage FDA procedural tools like the 513(g), FDA Pre-Submission, and other interactive techniques to get our clients’ ideas to market faster, with more transparency, and with fewer headaches.
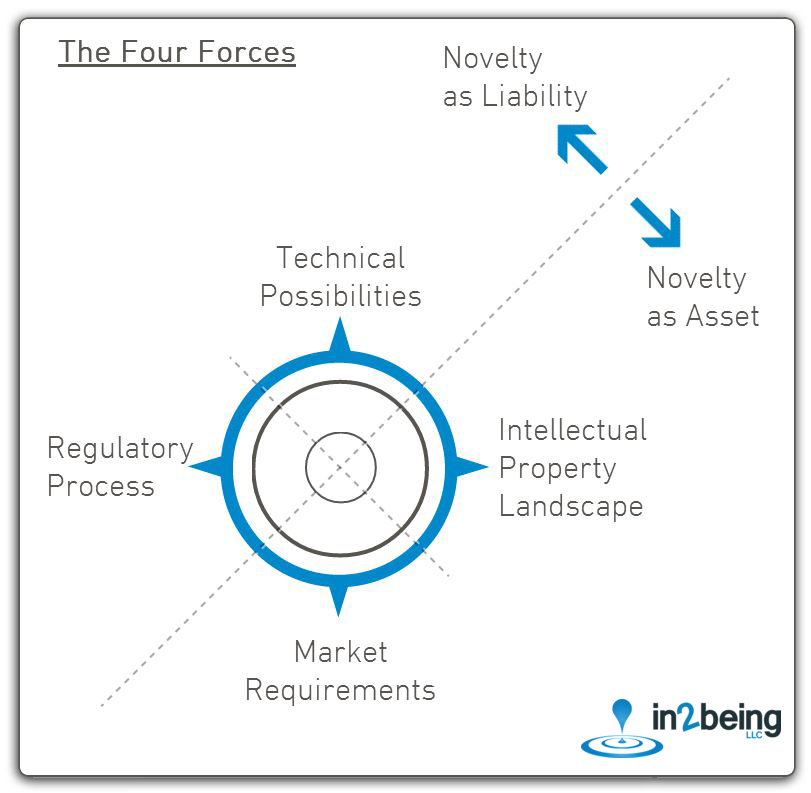
We focus intensely on creating needed balance between technical possibilities, market requirements, regulatory processes, and the often-crowded intellectual property landscape.

Battle-Tested Leadership
Our leadership consists of veteran med-tech entrepreneurs who have successfully developed a unique process for keeping regulatory, market, intellectual property, and technical forces in balance.
We understand that you’re interested in bringing new medical tech to market, not wandering through a bureaucratic maze full of regulatory dead ends.
How in2being Can Help
As many companies can attest, putting engineers, marketers, and regulatory professionals in the same room is a recipe for frustration and delays. Each stakeholder rarely understands the perspective of the others.
The in2being team overcomes that frustration and eliminates delays using a holistic approach to the medical device design process. We cut through complexity and ambiguity to get right to the heart of challenges faced by companies trying to bring their ideas to life. We help companies reduce the average time it takes to bring a medical device to market.
Our development professionals are equipped and empowered to hear the voices of all stakeholders in the development process and have the know-how to make the development picture fit together for a successful, low-frustration outcome.
