In 2013, a medical technology startup with an idea for a novel treatment for aortic dissection contacted in2being for full-service medical device consulting. The client only had academic resources available. Therefore, they needed help with all the facets of medical device development.
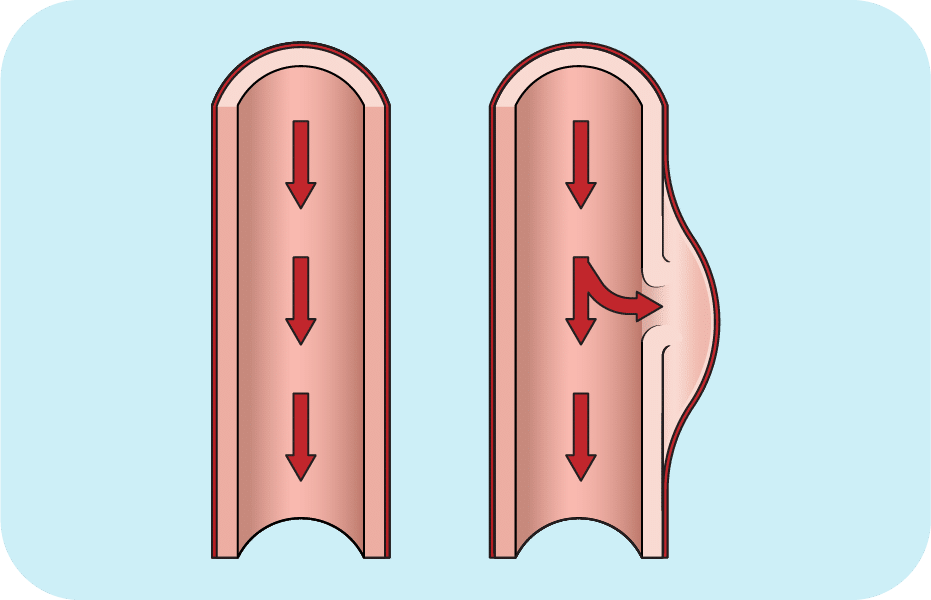
Aortic dissection is an uncommon but life-threatening condition in which the inner wall of the aorta—the body’s largest artery—tears. The tear (or dissection) allows blood to flow in between the inner and outer walls of the aorta, creating a false channel—a dead end. An aortic dissection can lead to an aortic rupture and internal bleeding, or it may cause malperfusion (reduced blood flow) to vital organs that leads, for example, to heart attack, stroke, or organ dysfunction.
Aortic dissections are categorized by their location: Type A in the upper section of the aorta, closer to the heart, brain, and other vital organs, and Type B in the lower section of the aorta. Type A aortic dissections require immediate treatment via direct surgical repair or an endovascular (inside the blood vessel) stent to cut off the false channel and reinforce the arterial wall. Type B aortic dissections are sometimes managed with medications (e.g., to reduce blood pressure) or are treated with endovascular stents.
The Starting Point: An Idea for a Novel Treatment
The client approached in2being with an idea for a novel device to treat aortic dissection.
A high mortality rate and limited treatment options have made aortic dissection a very active area of medical research. The client aims to develop a novel treatment modality for aortic dissection—a radiofrequency device.
However, because the client company is a startup, they have limited med-tech development resources and have availed themselves of the full range of in2being’s medical device development services.
The Process: Full-Service Consulting
During our lengthy engagement with this client, in2being has essentially served as their development team, providing the following services:
- Electrical and mechanical design: Working with the client’s input, our engineers produced electrical and mechanical designs for the radiofrequency treatment device.
- Design controls: in2being developed design controls for the device to comply with the current good manufacturing practices (CGMPs) required by the Food and Drug Administration (FDA) under the quality system regulation (QSR).
- Prototyping: We developed several generations of prototypes for the radiofrequency device.
- Vendor management: To help the client move toward manufacturing readiness, we’ve provided vendor management services, initiating and developing relationships with parts and materials suppliers.
- Testing: in2being carried out bench testing as the device was in development and later conducted animal trials.
- Regulatory consulting: in2being has also advised the client on appropriate strategies for FDA approval and prepared FDA regulatory submissions on the client’s behalf.
Throughout this engagement, we’ve also provided project management services, helping the client move forward toward regulatory approval, clinical trials, and manufacturing readiness.
The Result: Awaiting Clinical Trials
Currently, a clinical trial of the client’s device is pending, and we’re working with the client and their clinical trial partners to ensure that the trial gets off the ground and is successful.
in2being is a full-service medical device development consultancy with a proven track record of assisting clients with bringing new medical devices to market. Contact us today to learn more about how in2being can help you make your innovative med-tech ideas a reality.