A university research team contacted in2being in 2019 with an idea they’d developed for a novel angioplasty device. Although their innovation warranted further development, they didn’t have the resources they needed in the university environment.
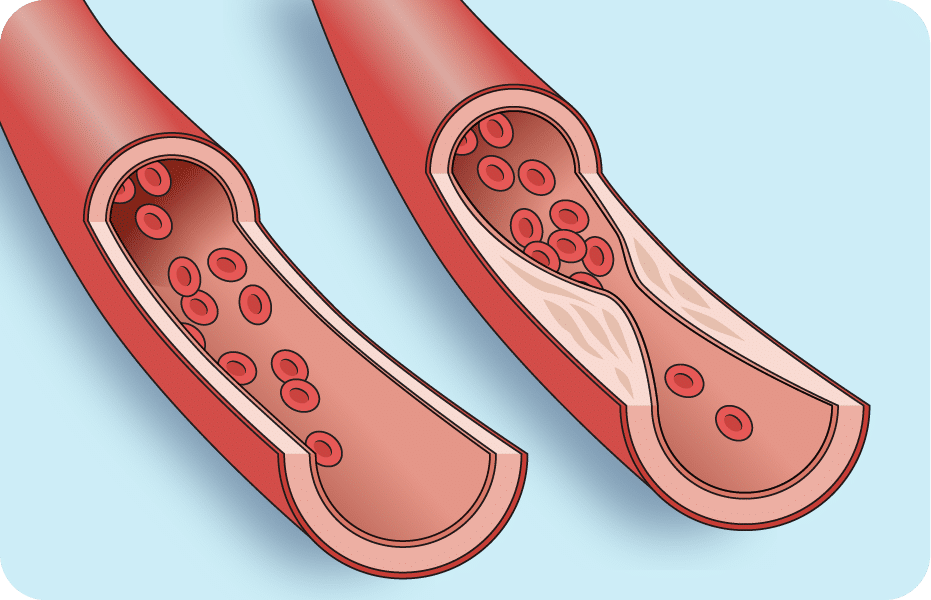
Atherosclerosis is a medical condition in which cholesterol, lipids, and calcium deposits called plaque build up in arteries. This condition can lead to blocked arteries, thromboses (blood clots), or aneurysms (weakened, bulging arterial walls). Symptoms and consequences vary depending on the arteries affected. For example, if atherosclerosis affects the coronary arteries, it can lead to heart attacks. When it restricts blood flow to the head or limbs, it’s called peripheral artery disease.
Angioplasty is an effective treatment for atherosclerosis in many cases. A thin, flexible catheter with an uninflated balloon on its end is inserted into an artery narrowed by atherosclerosis. The balloon is manipulated to the blockage and inflated, opening up the artery to allow greater blood flow. The balloon is driven by a base system that in2being helped to create.
However, in some cases, atherosclerosis can’t be treated with traditional angioplasty because the plaque deposits are hardened with calcium. The university research team wanted to develop an innovative angioplasty device that would be able to clear such calcified deposits in patients with peripheral artery disease.
The Starting Point: Solid Research
Although the university team had solid research and a viable concept, they needed help progressing toward a prototype.
In the first phase of our engagement, we began the design process with work on their design history file (DHF) to meet the requirements of the Food and Drug Administration’s (FDA’s) quality system regulation (QSR) for medical devices. This process included the following elements:
- Design plan
- Design inputs and outputs
- Design and document controls
- Preliminary risk/hazard analysis
- Software requirements
Additional services in2being provided in this phase included:
- Helping prepare an FDA pre-submission to solicit feedback on the proposed device
- Initiating interactions with potential contract manufacturers
- Advising the client on startup formation
The Process: From Research to Readiness
After the first phase of our engagement, the university team secured its first round of funding, formed a spin-off startup company, and continued working with us to develop their novel angioplasty device.
In the project’s second phase, in2being provided the following services:
- Mechanical and software design: We assisted the client in producing detailed mechanical specifications and operating software for the device, working toward manufacturing readiness.
- Prototyping: We produced early prototypes of the device.
- Verification testing: We conducted verification testing to ensure that we built the device properly.
- Vendor management: We developed and managed relationships with potential suppliers of parts and materials for the client device.
- Regulatory consulting: We continued to advise the client on FDA strategy as they worked toward a regulatory submission.
Because this was the client’s first foray into medical device development, we provided guidance and project management services throughout.
The Results: Funding and First-in-Human Trials
At the time of this writing, we’re still working with this client to bring their ground-breaking angioplasty device to market—and we’ve made great progress. In fact, in2being is still partnering with them as they continue to grow with over 20 employees! In 2022, they successfully completed a first-in-human trial of the device and secured more than $20 million in another round of funding. We’re currently assisting them in preparing an FDA regulatory submission.
New medical technology often starts with a breakthrough in research or a moment of sudden insight. But what do you do next? in2being’s mission is to help med-tech innovators bring their ideas to life with full-service medical device development consulting.
Contact us today to learn how we can help make your medical innovation a marketable reality.