FROM: Concept device
TO: Clinical trial ready devices, regulatory filings
In 2017, a university team engaged in gastroenterology research contacted in2being for help with an idea for a device that would help physicians screen patients for anorectal problems underlying their chronic constipation.
Functional constipation—defined as difficulty or delays in passing stool without an apparent underlying cause—is quite common. Researchers estimate that chronic constipation affects as many as 1 in 5 people worldwide. Frontline remedies for functional constipation are dietary and lifestyle changes (e.g., increasing dietary fiber, staying sufficiently hydrated, walking after meals) and over-the-counter laxatives (e.g., polyethylene glycol).
The problem is that these treatments will not necessarily resolve underlying physical issues. One study, Development of a Simple, Point-of-Care Device to Test Anorectal Function in Patients with Constipation: Randomized Clinical Trial, reports that “more than 95% of patients continue to take only over-the-counter laxatives and receive empirical dietary advice, whereas fewer than 2% undergo a physiologic evaluation to ascertain the cause of their symptoms.”
For example, one such cause is dyssynergic defecation, a disorder in which the pelvic muscles don’t coordinate properly to expel stool. It affects up to 25% of patients with chronic constipation. The condition is treatable, but diagnosis requires testing. Because few clinics perform the necessary tests, testing is scarce and expensive.
To address this gap in the diagnosis of defecatory disorders, the university research team developed a diagnostic catheter that primary care physicians (PCPs) can use to screen for them.
The Starting Point: Rubber Gloves and Cotton Balls
When they contacted in2being, the university team had been trying to create a prototype of their novel medical device using rubber gloves and cotton balls. They needed the design, engineering, and manufacturing resources that in2being provides to bring their idea to life.
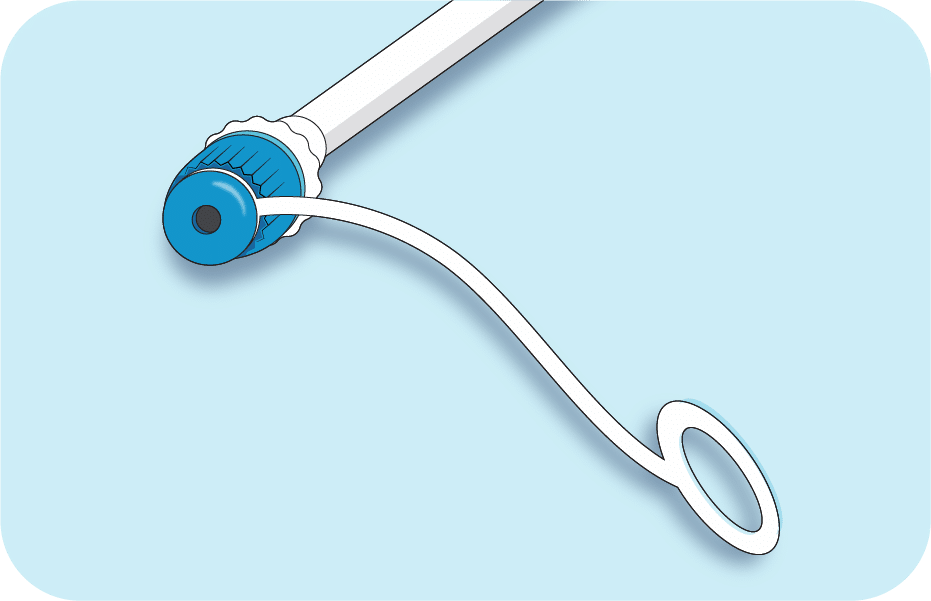
One primary test used to diagnose defecation disorders is the balloon expulsion test (BET). A doctor inserts a tube with a balloon on its end into the patient’s anus. Using a syringe, the doctor then inflates the balloon with water. The patient is given a stopwatch and asked to time how long it takes to expel the balloon.
The university team wanted to develop a simple, all-in-one device that would mimic the BET and enable a PCP or nurse to screen for defecatory disorders and justify referral to third-party testing facilities.
The Process: Full-Service Medical Device Development
The university team needed help with proof of concept, prototyping, and developing a manufacturable device.
in2being assisted in the following areas:
- Prototyping: in2being designed and created early prototypes of a device that simplifies the BET process for PCP office use. A healthcare practitioner inserts the catheter into the patient’s anus. Then, they inflate the stool-shaped balloon on the end by removing the cap on the end of the tube.
- Proof of concept: Using the prototype in2being developed, the university team conducted a successful proof-of-concept study with 20 patients.
- Manufacturing design and readiness: To help the client reach manufacturing readiness, in2being handled the following tasks:
- Mechanical design
- Development of quality systems and design controls
- Verification testing
- Vendor management
- Patent applications: in2being helped the university team prepare and file a patent application to protect its intellectual property.
- Regulatory filings: in2being assisted the client in preparing and submitting Food and Drug Administration (FDA) filings to confirm the diagnostic catheter’s medical device classification.
in2being also provided project management and trained the university team on the medical device development process.
The Results: Readiness for Clinical Trials
With in2being’s full-service medical device development services, the university team’s ideas for a novel diagnostic catheter have come a long way—from a crude initial prototype to patented technology ready for manufacturing and clinical trials. in2being’s work with this client is ongoing, so stay tuned for further developments.
If you’re a medical technology innovator with ideas for a new medical device, in2being’s comprehensive med-tech consulting services can take your project from concept to completion.
Contact us today to discuss your project and learn more about how we can help.