FROM: Napkin sketch
TO: 510(k) clearance, manufacturing readiness, patents
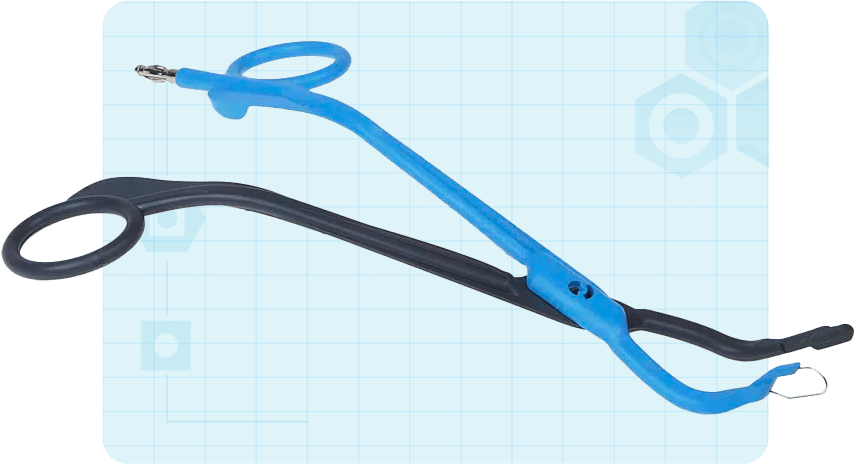
In 2013, a med-tech start-up founded by an accomplished ear, nose, and throat (ENT) surgeon contacted in2being with an idea for a novel surgical forceps for tonsil and adenoid removal.
The tonsils and adenoids are part of the lymphatic system, which removes excess fluid from bodily tissues and circulates it back to the bloodstream. The lymphatic system also aids in immunity by producing disease-fighting blood cells called lymphocytes. Adenoids are larger during childhood and shrink with age. They do not have a known function in adulthood.
Tonsillectomy or adenoidectomy is indicated when:
- Tonsils or adenoids become infected and swollen, interfering with breathing.
- Chronically swollen adenoids (in children) prevent the ear canal from draining, resulting in frequent ear infections.
- Tonsils (in adults) contribute to sleep apnea.
The Starting Point: A Napkin Sketch
The client came to us with a literal napkin sketch of an electrosurgical forceps for tonsillectomy and adenoidectomy.
The client wanted to develop a device that would use radio frequencies to remove tonsil or adenoid tissue at a lower temperature than other electrosurgical devices, resulting in less collateral tissue damage.
However, because the client did not have the resources to develop the medical device, they took advantage of the full breadth of in2being’s med-tech consulting services to bring the product to market.
The Process: Full-Service Consulting
During in2being’s three-year engagement with the client (2013–2016), we provided the client with full-service medical device development consulting that included the following steps:
- Proof of concept: We compiled a document that demonstrated the viability of the client’s ideas. The proof of concept document aided the start-up in attracting investors and validated continuing the development process.
- Intellectual property: We assisted the client with IP disclosures to secure patent applications to secure their intellectual property.
- Electrical and mechanical design: Our engineers took client ideas and input and used them to produce detailed electrical and mechanical plans for the electrosurgical forceps.
- Early prototypes: In our labs, we took the design from paper to working prototypes.
- Design controls: We developed design controls for the electrosurgical tool that complied with the current good manufacturing practices (CGMPs) required under the Food and Drug Administration’s (FDA’s) quality system regulation (QSR). This step included compiling and maintaining the device’s design history file (DHF).
- Verification and validation testing: Following the FDA’s good laboratory practices (GLPs), we carried out design verification and validation testing to ensure that we were building the forceps properly and that they would work as intended.
- Manufacturing prototypes: We collaborated with local vendors to ramp up manufacturing.
- Animal testing and trials: To gather data for the FDA clearance process, we conducted tissue testing, animal testing, and other verification testing.
- 510(k) clearance: The change led to accompanying the client through the FDA’s designated regulatory pathway for Class II medical devices, a 510(k) submission.
As the development process progressed, we also provided project management services, assisted with vendor management, and addressed other facets of manufacturing readiness.
The Results: Approved Patents, FDA Clearance, and Manufacturing Readiness
By the end of our work with the client in 2016, we had shepherded their novel ENT surgery tool from napkin sketch to market readiness. Our medical device consulting services provided this med-tech start-up with:
- Intellectual property protected by approved patents.
- 510(k) clearance, allowing them to legally market their new medical device.
- Everything in place to begin manufacturing their device.
If you’re a medical technology innovator, in2being can help you get from concept to market. Whether you need full-service consulting or an assist in one area of development, contact us today to learn how we can bring your medical device ideas fully into being.